Introduction
Type 1 diabetes mellitus (DM1) is one of the most common childhood chronic diseases, but it can occur at any age. Autoimmune infiltration leads to the destruction of beta cells and a partial, subsequently complete, deficiency of insulin and, as a result, the absolute necessity of its substitution. The state of insulin deficiency leads to the development of a complex metabolic disorder characterized by chronic hyperglycaemia [1]. Children with DM1 need special attention and systematic paediatric care [2]. They are a high-risk group for accelerated atherosclerosis, because of which chronic microvascular (retinopathy, nephropathy, neuropathy) and macrovascular (myocardial infarction [MI], peripheral arterial disease [PAD], stroke) complications may occur [3].
Oxidative stress (OS) is associated with the pathogenesis of diabetic vascular complications. It is postulated that hyperglycaemia induces reactive oxygen species overproduction and a decrease in the endogenous antioxidant defence systems through different mechanisms such as increased formation of advanced glycation end-products (AGEs), activation of protein kinase C (PKC), and hyperactivity of the hexosamine and sorbitol pathways. The overproduction of reactive oxygen species (ROS) is very harmful to the cell. It causes oxidative damage of the cell structures, modulates intracellular signalling pathways, increases expression of pro-inflammatory and pro-coagulant factors, induces apoptosis, and impairs nitric oxide release. Furthermore, fluctuations of blood glucose levels (glucose variability) are also associated with OS. Oscillating glucose compared to chronic hyperglycaemia can have even more deleterious effects on endothelial function and oxidative stress. Recent studies suggest that hypoglycaemia is also an important factor in cardiovascular damage through OS, catecholamine release, inflammation response, platelet activation, pro-thrombotic events, and endothelial dysfunction [4].
The aim of this review was to summarize the current literature on the correlation of glycaemic control and the development of atherosclerosis and the incidence of cardiovascular disease (CVD) in young people with DM1.
Targets for glycaemic control
Self-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM), along with the assessment of glycosylated haemoglobin (HbA1c) levels, are the tools used for monitoring diabetes treatment worldwide. International and national glycaemic control targets differ between guidelines due to variable access to, and availability of, diabetes care and supplies [5, 6]. According to recent Diabetes Poland guidelines, the goal of glycaemic control in patients with DM1 is to maintain fasting and a pre-meal glycaemia level of 70–110 mg/dl (3.9–6.1 mmol/l), and then < 140 mg/dl (7.8 mmol/l) 2 hours after the start of the meal. In children and adolescents, regardless of the type of diabetes, the HbA1c target was set at ≤ 6.5%. The rapid development of new technologies and increasing evidence of limitations in HbA1c and SMBG, such as lack of information about the size and frequency of inter- and intraday glucose fluctuations, have contributed to the more common use of CGM systems [6]. They provide up-to-date information on the concentration of glucose in the interstitial fluid, making it possible to observe its recent changes and to predict the behaviour in the near future (the so-called “trend”) [6, 7].
The analysis of data from CGM reports (minimum 70% of sensor use) allows determination of the key metrics: mean glucose concentration, glycaemic variability (GV), time in range (TIR), time above range (TAR), and time below range (TBR). The coefficient of variability (%CV) is considered the metric of choice to describe intraday GV [6, 8]. The dissemination and effectiveness of CGM systems resulted in the development of recommendations indicating the target values of the parameters mentioned above (Table I) [6, 7].
Table I
Target values of time in range (TIR), time below range (TBR), time above range (TAR), and coefficient of variability (CV) in people with DM using CGM systems [6, 7]
Cardiovascular risk in type 1 diabetes mellitus
The main cause of death in people with DM1 is CVD [6]. It is associated with atherosclerosis, which is a long-term process that begins early in life in everyone but develops much faster in people with DM1 [3, 9].
Compared with children without DM1, peers with DM1 have an increased risk of developing advanced atherosclerosis, which is directly related to the occurrence of CVD [3]. In people with DM1, the risk of CVD increases up to 10 times, cardiovascular events are more frequent, and they occur earlier than in healthy people. In comparison to DM2, in DM1 CVD is revealed at a younger age, and atherosclerosis is more diffuse and concentric [10]. Although macrovascular complications are rarely manifested in childhood, the features of subclinical atherosclerosis can already be stated as their prediction [3].
Childhood and adolescence are considered critical stages of life in predicting the risk of vascular complications in DM1 [10]. A longer duration of the disease, especially in the first decades of life, may exacerbate the adverse metabolic effect of DM1 on blood vessels and accelerate the onset and development of atherosclerosis [9]. In the group of people with DM1 registered in the Swedish national registry, a correlation between the age of onset, CVD risk, and lifetime was indicated. The onset of DM1 in the first decade of life shortened life expectancy by 17.7 years for women and by 14.2 years for men, which is 7.6 and 4.8 years more, respectively, than when DM1 was diagnosed at the age of 26–30 years. The risk of CVD among people who developed DM1 before the age of 10 years increased 30 times [11]. These conclusions indicate that interventions aimed at preventing and slowing the development of CVD – such as optimal glycaemic control from DM1 onset – are most effective if implemented at a young age [2].
DM1 is an independent risk factor for CVD; however, additional accompanying CVD risk factors accelerate atherosclerosis, including age, HbA1c values, disease duration, presence of hypertension, proteinuria, obesity, dyslipidaemia, and lifestyle factors (e.g. lack of physical activity or nicotinism). Some of the above-mentioned CVD risk factors are more common in children with DM1 than in the general paediatric population, especially in girls [10]. The relationship between CVD risk factors in DM1 during childhood and vascular abnormalities in adulthood was investigated in some scientific papers [3, 10]. The current recommendations suggest starting screening for macrovascular complications in children and adolescents with DM1 soon after diagnosis from the age of 11 years. Screening methods include annual BP measurements and lipid profile every 2 years [12].
Chronic hyperglycaemia is the primary mediator of atherosclerosis in DM1, and it increases the risk of developing CVD; therefore, optimal glycaemic control helps restrain preclinical abnormalities in endothelial function in adolescents with DM1 and is crucial to avoid chronic complications and premature death [3, 6].
In the multicentre DCCT study of a group of 1441 young DM1 patients treated with intensive insulin therapy, a lower percentage of early microvascular complications was observed compared with the conventional treatment group. The long-term follow-up (EDIC study), during which all DCCT patients were treated with intensive insulin therapy, showed that people with initially better glycaemic control during DCCT, despite similar glycaemic outcomes to the control group in the following years of the disease, presented lower frequencies of both microvascular and macrovascular complications. Moreover, the positive effects were maintained despite less rigorous therapy [13].
Also, other risk factors apart from hyperglycaemia need to be considered. The prevalence of overweight in DM1 increases faster than in the general population. It is worth underlining that obesity, especially with associated insulin resistance factors, may negatively impact glycaemic control, but also may independently increase CVD morbidity and mortality [6].
Assessment methods of atherosclerosis
Several noninvasive imaging techniques are used in clinical practice for the assessment of early vascular abnormalities. The main indicator of the development of atherosclerosis is endothelial dysfunction, which can be defined by tests like flow-mediated dilatation (FMD), pulse wave velocity (PWV), and an increase in the artery intima–media thickness (IMT) in almost all arteries [14]. Another metric for atherosclerosis, specially used for diagnosis of PAD, is ankle-brachial index (ABI) [15].
Carotid artery intima and media thickness
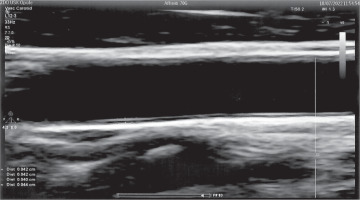
Carotid artery intima and media thickness (cIMT) is considered a structural marker of early atherosclerosis in DM. It depends on metabolic control and increases as the disease progresses, predicting several macro- and microvascular complications [2]. The IMT is measured as the distance between the lumen-intima and media-adventitia interfaces (Fig. 1). The presence of atherosclerotic plaques in the carotid artery is a strong predictor of CVD [15]. Many research papers have used the assessment of cIMT by ultrasound scan (USG) as one of the best methods due to its non-invasive nature, easy accessibility, and high accuracy. USG visualizes the structure of the arterial wall with better resolution than any other imaging. Due to its increasing popularity, as well as the growing number of studies using cIMT measurements, it was necessary to unify the guidelines for the cIMT assessment technique. Examination of the carotid wall should be performed using a high-resolution B-mode system equipped with a linear array transducer > 7 MHz [16].
The first cIMT consensus took place in Mannheim in 2004, and the latest update was in 2011. The paper describes examination procedures in detail. The main cIMT scanning and reading protocol recommendations are as follows: 1. IMT measurement should be performed in a plaque-free region. 2. Measurements can be made in the common carotid artery, carotid sinus, and internal carotid artery. 3. In research studies assessing the thickness of vessel walls, the values obtained from different measurement sites should be documented separately [16].
The cIMT > 0.9 mm is considered too thick, but the highest valid value varies with age (Table II). IMT ≥ 1.5 mm suggests the presence of atherosclerotic plaque [15].
Table II
Mean values of cIMT in children and adolescents in the age group 10–20 years. Adapted from [17]
| Age [years] | 10–13 | 14–16 | 17–20 |
|---|---|---|---|
| IMT in girls [mm] | 0.38 ±0.04 | 0.40 ±0.04 | 0.39 ±0.03 |
| IMT in boys [mm] | 0.38 ±0.03 | 0.39 ±0.05 | 0.40 ±0.03 |
Flow-mediated dilatation
Flow-mediated dilatation is considered to be a measure of nitric oxide bioavailability, and therefore endothelial function. Flow-mediated dilatation is measured after transient vascular occlusion to assess the ability of an artery to expand due to ischaemia. The reduction in FMD indicates endothelial dysfunction [14]. Flow-mediated dilatation assessment is useful in predicting future cardiovascular events. This test can be performed in various places of the peripheral circulation. In the case of the brachial artery, the diameter of the resting artery and the blood flow velocity are measured. Subsequently, the sphygmomanometer cuff is placed over the proximal arm or distal forearm and inflated to a value of 250 mmHg for 5 minutes. The maximum vessel diameter is measured at the 60th second after the pressure is released. Artery diameter measurements should be synchronized with the R wave on the electrocardiogram [18].
Formula 1. Formula for FMD, where A = diameter in 60 seconds after ischemia; B = diameter before ischaemia [18].
Pulse wave velocity
Non-invasive PWV assessment is another marker of vascular damage. It is a measure of the stiffness of the arteries. During left ventricular contraction, the stroke volume reaches the aorta, widening its wall and creating a pressure wave that travels distally towards the peripheral tissues. The stiff aorta is unable to absorb the pulsed wave – the stiffer the aorta, the faster the pulse wave becomes. This causes vascular remodelling and secondary organ damage. In addition, the pulse wave bounces back and returns to the heart, increasing blood pressure and stress on the heart, and by reducing coronary perfusion it increases LV hypertrophy [14]. The PWV value of the examined artery sections is calculated thanks to special algorithms. PWV > 10 m/s is considered abnormal [15].
Ankle-brachial index
Ankle-brachial index is used to assess peripheral atherosclerosis. It can be measured with a continuous wave Doppler unit and a BP sphygmomanometer. The ABI is performed by measuring SBP from both brachial arteries and from both the dorsalis pedis and posterior tibial arteries with a handheld Doppler instrument. The ABI is calculated for each leg using a mathematical formula (Formula 2). A low ABI (i.e. < 0.9) indicates lower extremity artery disease (LEAD) [15].
Formula 2. Formula for ABI, on the example of the right side [15].
The influence of glycaemic control on atherosclerotic vascular changes
Studies concerning the prevalence of subclinical atherosclerosis in young people with DM1 are limited. In a population-based study in 2010, 314 children and adolescents with DM1 (mean of age 13.7 years and mean diabetes duration 5.5 years) had cIMT measured and compared with 118 age-matched healthy control subjects. 19.5% of diabetic patients were above the 90th centile of healthy control subjects, and 13.1% were above the 95th centile [10]. Research from 2017 included 1746 children and adolescents with DM1 (mean age 17.9 years). The results showed that 11.6% of subjects had a PWV in the 90th centile or above [19]. Another study tested 289 asymptomatic adults with DM1 using ABI and detected abnormal values (≤ 0.9 or > 1.2) in 92 patients (32%). The result was confirmed by the toe-brachial index (TBI) and peripheral Doppler ultrasound (DUS) in 37 subjects, resulting in a 12.8% prevalence of asymptomatic PAD [20].
As previously mentioned, data show that glycaemic control remains significantly associated with cIMT and incidence of carotid plaques after adjusting for age, gender, statin treatment, and other risk factors for CVD. This correlation is observed in DM1 in all stages of carotid atherosclerosis – from preclinical, through early symptoms visible in IMT, to advanced vascular disease [10]. Increased cIMT was found in the paediatric population with DM1 compared with healthy controls [2, 3, 10].
For DM1, positive correlations were found between cIMT and disease duration, body mass index (BMI), blood pressure, heart rate, LDL concentration, and daily insulin requirements [21]. In children with DM1, it was observed that treatment with a personal insulin pump led to a decrease in both HbA1c and cIMT [22].
Only a few studies have described the association between methods assessing atherosclerosis and CGM parameters. In adolescents (14–17 years old) with DM1 and insufficient glycaemic control, HbA1c, total cholesterol, and LDL correlate with increased PWV. However, the relationship between PWV and the daily insulin dose, disease duration, gender, or SBP has not been proven [23]. To the best of our knowledge, no more studies in paediatric population have addressed this topic so far. Most papers concerned the adult population, often with DM2, and their results were inconclusive.
A study in adults with DM1 showed that TAR and the glucose management indicator (GMI), which indicates the average HbA1c level and is derived from 14 days of CGM data, were directly associated with the presence of microvascular complications, while TIR had an inverse relationship. TBR was directly associated with the presence of plaque, which shows the role of hypoglycaemia in the development of CVD in the population with DM1 [24].
In a paper analysing data from 2215 adult patients with DM2 it was proven that TIR is associated with cIMT. After eliminating the impact of well-known risk factors of CVD, each 10% increase in TIR was associated with 6.4% lower risk of abnormal cIMT. The relationship between TIR and cIMT remained significant, regardless the status of microvascular complications [25].
On the other hand, the authors of one study described higher values of cIMT and lower FMD in people with DM1 compared with healthy controls, but no correlation of both parameters with the values of TIR, TBR, TAR, or mean glycaemia was confirmed [26]. Further studies also did not show a significant correlation of CV with cIMT [21]. In adults with DM2, in the fully adjusted models for demographics, cardiovascular risk factors, and lifestyle factors, both higher CV and lower TIR were significantly associated with higher carotid to femoral pulse wave velocity (cf-PWV). In the same study, a correlation between CV and ABI was not confirmed, and only TIR ≥ 70% was independently associated with ABI [27]. It seems that among the parameters derived from CGM reports, only CV and mean amplitude of blood glucose spikes (MAGE) are strongly associated with susceptibility to plaque formation in DM1 [28]. Research on the adult population suggests that arterial stiffness as a parameter correlates with mortality in people with DM1 [29]. As recommended by the American Diabetes Association, regular evaluation of CVD risk factors should be performed annually in all patients with DM [30].
Conclusions
Type 1 of diabetes mellitus is an independent risk factor for CVD; moreover, in the development of atherosclerosis hyperglycaemia, glycaemic variability and hypoglycaemia are important modifiable risk factors. The use of CGM systems allows for more precise glycaemic control and its analysis, as well as making more effective therapeutic decisions. However, the relationship between the parameters obtained from CGM reports and the risk of CVD in DM1, especially in children, has not been clearly established so far. The results of the conducted studies on the correlation of CGM parameters with measurements indicating subclinical atherosclerosis are inconclusive, and moreover, they focus mainly on the adult population. Therefore, there is a need to extend the knowledge in this area and conduct further longer-term studies, especially in the paediatric population, where the initial years of the disease have a significant impact on the development of long-term complications of DM1.

 POLSKI
POLSKI