Introduction
Obesity is a health crisis with adverse health effects worldwide. In 2005, globally, the 33% prevalence rate of (1.3 billion people) overweight and obesity was estimated to reach 58.8% (3.3 billion people) by 2030 [1]. Concern over obesity development from childhood to adolescence has been raised [2]. The global burden of the body mass index (BMI) group of WHO suggests higher weight gain prevalence as a greater risk factor than undernutrition worldwide [3]. The rise in industrial activity was reported to cause a rise in obesity prevalence due to the accumulation of chemicals (endocrine-disruptive) in the human body linked with the obesity epidemic [4]. Adverse effects of toxic chemicals on lipid metabolism and adipogenesis have been reported [5] and termed obesogens. Bisphenol A (BPA), polyfluoroalkyl chemicals (PFCs), polybrominated biphenyl ethers (PBDEs), organochlorine (OC) pesticides, polychlorinated biphenyls (PCBs), some solvents, and phthalates have been reported as possible obesogens causing weight gain and neural sensitivity [6]. Concern over bisphenol A occurrence in the environment has been increasing because it is ubiquitous in resins and plastics to protect food, and being a well-known endocrine-disruptive chemical (EDC).
“There is no packaging that is perfect”, and there is a rising trend of using packaged/processed food in both developing as well as developed countries. These compounds packed in different kinds of containers comprise “plastic bottles, foil retort pouches, glass jars, jar lids, metal cans, paperboard boxes with polyethylene liner bags, liquid paperboard, greaseproof wrappers, offset migration, and microwave use” [7].
In previous studies disorders related to picky eating behaviour among children [8] and binge-eating disorders among college students [9] have been reported. Such eating behaviours can be influenced by the various dietary and non-dietary exposure sources, which might be linked to the picky eating behaviour. In another study, habituation and reinforcing the value of food have been linked with energy intake and body weight [10]. Similarly, disordered eating was linked with mean R wave amplitude, a cardiac biomarker [11].
Bisphenol A and heavy metals are endocrine disruptors. When heated to high temperatures, these products tend to be released from the material, and they are commonly used in consumer products, such as food cans, soda bottles, polyester-styrene, polycarbonate plastics, and baby bottles [12, 13]. The presence of bisphenol A has been reported in thermal printing papers, cash register receipts [14], protective coatings, flame retardants [15, 16], supply pipes, water storage tanks [17], some dental sealants [18], and fillings [19].
According to the U.S. and European authorities, the evidence is increasing for bisphenol A and heavy metals as emerging endocrine-disruptive chemicals, which warrants the removal of chemical migration from food contact materials [20, 21]. The USA has abandoned the manufacturer and use of bisphenol A in baby bottles, infant-formula packaging, and baby bottles [22], as well as other countries: Canada, Malaysia, China, South Africa, the European Union, Argentina, Ecuador, and Brazil [23]. Similarly, France has recently implemented a ban on bisphenol A that comes into contact with food [24].
The mechanism underlying the role of toxic chemicals in childhood obesity
Heavy metals, bisphenol A, phthalates, and some solvents are reported to increase weight gain by controlling the lipid homeostasis resulting in developmental programming and obesity [25]. Exposure to endocrine-disrupting chemicals in early life may have adverse health effects [26], including succeeding generations [27]. Obesogens can affect leptin, ghrelin, and inhibiting aromatases [28, 29] or through modification of the expression mechanism [30]. Exposure to obesogen has been reported to alter the serum levels of metabolic hormones, leading to an increase in weight gain [30] and an effect on pro-inflammatory cytokines [31–33], leading to metabolic syndrome and diabetes in paediatric age groups. The influence of chemical exposure on intrauterine growth retardation, low birth weight, and prematurity are documented as risk factors for obesity [34–36]. There are a growing number of epidemiological links between environmental exposure and obesity. However, rapid changes in lifestyle habits, increased energy intake of fast food, and decreased physical activity are considered the leading causes of weight gain.
Material and methods
To date, relatively few studies have reported the obesity association with BPA and heavy metals. It is evident that bisphenol A and heavy metals are endocrine-disruptive chemicals present in our lifestyle. This raises the question about health risks because these compounds pose childhood health problems. The present study determines bisphenol A and heavy metals in biological fluid (urine and blood) using LC-MS/MS and ICPMS. The current study was designed to assess the exposure level of bisphenol A and heavy metals among North Indian non-obese, obese, and underweight children. Individual and combined association between urinary bisphenol A and heavy metals will also be assessed among non-obese, obese, and underweight children. A hypothesized model will be designed to evaluate the exposure pathway from different dietary and non-dietary exposure causes of bisphenol A and heavy metals from North India.
Setting
Study participants will be included from the nearby community as well as the Paediatric Endocrinology/obesity Clinic, Paediatric Growth Lab, Paediatric Biochemistry Lab, Advanced Paediatric Centre, and PGIMER Chandigarh India.
Sample size estimation
We hypothesized that the mean bisphenol A levels in normal-weight children would be 2.8 ng/ml (a cut-off level in the US children for 2nd quartile bisphenol A levels) and 5.4 ng/ml (a cut off level for 4th quartile in US children) for the present study [37]. Their corresponding SD values of 6 and 8 were taken from Indian data [38]. Considering an alpha level of 5% and power of 80%, we calculated the required sample size of 93 children (rounding to 100) in each group will be recruited, giving us a total sample size of 300 children.
Participants
Children aged ≥ 6 – ≤ 16 years and measurement of BMI percentile for the age of CDC 2000, growth standards. Controls will be age-matched non-obese (normal and overweight) children with BMI ≥ 5th and < 95th percentiles and underweight < 5th percentile according to age and sex-specific 2000 CDC Growth Charts following the enrolment strategy (Fig. 1). Any acute infection, hereditary or systemic inflammatory disease, inborn errors of metabolisms and genetic disorders associated with dyslipidaemia and hypertension, and diabetes/regular medications were excluded from the study.
Sample preservation
Blood samples (5 ml) will be obtained after overnight fasting in clean and mineral-free tubes for heavy metal analysis, blood sugar, and lipid profile, as well as thyroid profile. Study participants will be asked to collect the morning urine samples in the container provided to them, and the containers will be stored at –80°C until the analysis by LC-MS/MS.
Study procedure
Anthropometric assessments will be conducted in the Paediatric Endocrinology clinic and Growth clinic of Advanced Paediatric Centre, PGIMER Chandigarh, India. Weight will be measured on electronic scales to the nearest 50 g with children barefoot and with minimal clothing. Height will be measured with a stadiometer to the nearest 1 mm. Body mass index (BMI) will be calculated as weight in kilograms divided by height in metres squared and expressed as kg/m2. Waist (above the belly button and below the rib cage) and hip (over the largest part of buttocks) circumference (WC and HC) will be measured to the nearest 0.1 cm by using standardized anthropometric techniques. The waist-to-hip ratio (WHR) will be calculated. Based on the BMI, children will be grouped as simple overweight (BMI ≥ 85th and < 95th percentiles) and obese (BMI ≥ 95th percentiles), non-obese (BMI ≥ 5th and < 85th percentiles), and underweight (BMI < 5th percentile). Informed consent from parents or primary caregivers and assent from the child will be taken before inclusion in the study. All the procedures followed during the study will be in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Socio-demographic characteristics
Family education level, occupation, and monthly income will be assessed as per the Kuppuswamy scale [39] because this scale is applicable in both rural and urban settings.
Laboratory assessment
Biochemical Investigations: Levels of glucose, total cholesterol (TC), HDL, LDL, VLDL, triglycerides (Tg), and hs-CRP will be quantified using an ADVIA 1800 clinical chemistry analyser (SIEMENS). The concentration of LDL-C will be calculated using the Friedwald formula as LDL-C = TC-HDL-(TG/5). Thyroid profile T3, T4, and TSH will be quantified by ADVIA Centaur XP chemiluminescence immunoassay.
Quantification of blood heavy metals: Inductively Coupled Plasma Mass Spectrometry (ICP-MS) validated multi-element method will be performed for metals analysis. Agilent 7700x ICP-MS with collision cell technology will be used, which operates on Helium (He) collision mode, to remove all polyatomic interferences using a single set of conditions, giving lower levels of interference and better long-term stability. Whole blood and serum samples (200 µl) will be taken, followed by the addition of 2 mL of nitric acid, 0.5 ml of hydrogen peroxide, and 10 µl of gold solution (10 µg/l) in a microwave digestion tube [40].
Quantification of urinary bisphenol A: In a glass tube, a urine sample (400 µl) and 10 µl internal standard (bisphenol A d6, 100 ng/ml) will be taken followed by the addition 5 µl of > 100,000 units of beta-glucuronidase enzyme and vortexed. Glass tubes will be placed in a water bath for 16 hours at 37°C. Methyl tertiary butyl ether (MTBE) 2 ml will be taken for liquid-liquid extraction. An upper organic layer will be taken and evaporated at 40°C. Finally, a dried sample will be reconstituted in a 200 µl buffer solution (mobile phase) and transferred to an autosampler vial [41]. A liquid chromatography-mass spectrometer (TMS) (ABSCIEX 4500) will be used to measure bisphenol A. The chromatographic separations will be carried out using an HPLC column equipped with a guard column. The HPLC system is coupled to a triple quadrupole instrument (API 4500; AB Sciex, Germany) equipped with a turbo electrospray source. The analyte is detected in positive-ion mode at a vaporization temperature of 500°C with an ion-electrospray voltage of 4.5 kV and analysed in multiple reactions monitoring (MRM) mode. Analyst software version (ABI Sciex) will be used in the handling of the data.
Exposure pathway assessment
Today, we all are surrounded by packaged food items, from consumer goods to foodstuffs. Although packaging prevents spoilage and works as a safeguard during transportation, sometimes these valuable containers may leach chemicals and other compounds, which may come into the food and drinks. Such contamination is ubiquitous in the environment and can happen in all types of packaging (e.g. plastic bottles, glass, and metal cans). Children at an early stage are prone to eases, and exposure to environmental chemicals may cause serious health consequences in the later stage of life. There are chances of such chemical exposure to any population in any stage of life through anthropogenic activity from application to crops, transport of chemicals, and food packaging to protect it from the external environment.
In this study, we developed a set of questions to assess the different exposure sources to bisphenol A and heavy metals as mentioned below.
Household characteristics: Housing is a significant risk factor for children to have exposure to bisphenol A and heavy metals. Efforts have been made in this study to assess the level of bisphenol A and heavy metals. The questionnaire includes queries related to the type of housing, construction type, source of lighting, whether a kitchen is separate or not, any wallpaper in the house, and crowding in the household. The physical quality of the home will be assessed to identify any structural defects that allow the entry of external chemicals into the household environment causing acute intoxication.
Surroundings: Here, we have tried to gather children surrounding area where they eat, live, play, drink, breathe and sleep. There is emerging evidence that air quality in the home, school, and coaching centres may impair children’s health [61].
Food and water: Special attention has been given to food, water, and utensils as a source of transportation by ingestion, inhalation, and drinking to children and impact on consumers, especially children. Focuses on heavy metals such as arsenic, lead, cadmium, mercury, and chromium were made. These heavy metals have been widely studied, and their roles in cancer of the bladder, lung, skin, kidney, and liver have been documented.
The children’s physical/dietary habits and nutritional assessment: Anthropometric examination is a mandatory tool to assess the child’s growth. Anthropometric measurements are useful in the nutritional assessment because each measurement depends on adequate nutrition. Poor growth in children indicates malnutrition. Physical measurements like body weight and height of children have been extensively used to define communities’ health and nutritional status. Based on the age, body weight, and height, many indices such as weight for age, weight for height, and BMI for age will be calculated. The interviewer prepared a food frequency questionnaire, a detailed questionnaire, including the list of foods and the child’s answers as to how often each food he/she eats per week, which will be noted down as exposure history.
Briefly, the following parameters will be used to assess exposure pathway: source: e.g. (water, air, plastic containers, utensils), pathway/exposure: e.g. (eating, drinking, breathing), receptor: e.g. (subjects) who are exposed or potentially exposed will be assessed for bisphenol A and heavy metals.
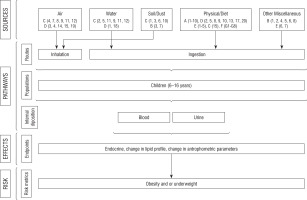
Measurements: Various exposure sources, uses frequency, and duration of use are explained in Table I. Based on the various exposure sources as listed in Table I, the major risk factor category (e.g. socio-demographic, air, water, diet, genetics, and miscellaneous) is explained with the description of major sources (Table II). This data will help us to develop a conceptual model (Fig. 2).
Table I
Exposure sources, frequency of use, and duration of use
Table II
Based on the above exposure pathway, the sub-heading questionnaire was categorized into their various exposure sources, e.g. air, water, diet, genetics, and miscellaneous
Statistical approach
The main objective of this study is to compare the urinary bisphenol A and heavy metals among obese, non-obese, and underweight children and their relationship with childhood obesity. To test associations, standard statistical methods will be applied using R version 4.0.0 and SPSS version 25 to analyse the data. All the data will be recorded in an MS Excel spreadsheet for future use.
Discussion
This study protocol planned to analyse the association between various dietary and non-dietary exposure sources to bisphenol A and heavy metals among North Indian children, and their relationship with obesity. Similarly, recently a study protocol has been published in which multi-disciplinary approaches among school-aged girls have been studied to weight management [42]. Previous studies reported a positive association between urinary bisphenol A and obesity in the USA [37] and Denmark [37, 43]. Similarly, nutrition and diet [44], genetic changes [45], and hormonal imbalance [46] were also reported as obesity risk factors [44] among children. Risks from socioeconomic status, education, gender [47, 48], residence area [49], physical inactivity [50], and watching TV [51] were also reported to be major contributors of obesity among children at an early stage of life. In this study, we planned to analyse the data using the implication of LASSO considering the multiple variables in the study. Similarly, in previous studies, generalized linear models for regression analysis [52] and LASSO were applied for shrinkage and variable selection to identify the chemical mixture components [53, 54].
Rural locality was reported to be associated with a lower body mass index [55]. Other studies suggest that urban areas are more prone to stress and depression, affecting health and the possibility of higher body mass index [56]. We also hypothesized that the role of the chemical mixture has an association with body mass index, because there is some emerging evidence [57, 58].
Levels of individual metals have been found to be associated with obesity. An increase in iron absorption after weight loss intervention among obese children has been reported [59]. Similarly, zinc has been associated with the developmental role of metabolic syndrome, regulating cytokine expression and suppressing inflammation [60].
A previous study shows that the Indian dietary pattern is highly diverse and gives evidence of an association between dietary pattern and health outcome of obesity as well as a cardiovascular disorders. Future work would benefit from using large food consumption data sets from different dietary and non-dietary exposure sources to study the range of Indian dietary patterns to identify the crucial links between exposure routes and disease.
While measuring emerging pollutant levels in biological samples, a cautious interpretation between fasting and non-fasting state samples is warranted because the associations are not always linear. Other risk factors can be linked to obese and undernourished children. The penalized regression approach shows multiple significant associations between the obese, non-obese, and undernourished categories of both fasting and non-fasting states. Qualitative assessment of dietary and non-dietary exposure sources must be included in future environmental or occupational health risk assessment studies for emerged and emerging pollutants.
Recommendations
Risk assessment studies could also be done in the form of a survey of bisphenol A concentrations in infant formula (if packed in metal cans), bisphenol A migration from recycled food packing paper, and biomonitoring studies in pregnant women to reduce bisphenol A exposure, because it is a well-known endocrine-disruptive chemical and is widely used.
Targeted intervention studies should be done for different exposure routes of bisphenol A and heavy metals from dietary and non-dietary sources because this will be helpful for the policymakers to implement the intervention to reduce the disease burden among children. Guidelines to minimize the bisphenol A and heavy metals exposure should be formulated as a preventive measure. Policymakers and local bodies (school, individual, and household level) should educate the population about emerging pollutants and their risks. Recycling codes 3 or 7 indicate the presence of bisphenol A, and consumers should be taught ‘how to read’ the bottom of the products to assess bisphenol A presence in different chemical migration sources. A warning to the users to avoid boiling liquid in containers with bisphenol A should be appropriately displayed.
Availability of data and materials
The spreadsheets generated from this study are not publicly available due to the need to protect the participant’s privacy. However, the quantitative data of urinary bisphenol A and heavy metals from North Indian children will be published.
Ethics approval and consent to participate
The internal Review Board of the Postgraduate Institute of Medical Education and Research, Chandigarh approved all study procedures. Written informed consent from parents and assent from children for their participation in the study at the time of enrolment from each participant will be taken care. PGIMER, Chandigarh Ethics Committee Approval Number: NK/3639/PhD/8326.

 ENGLISH
ENGLISH