Introduction
It is known that the treatment of type 1 diabetes is a challenge for both the patient and the doctor. Despite the constant struggle, most patients are unable to achieve normoglycaemia and are at risk of episodes of hypoglycaemia, diabetic ketoacidosis, or long-term vascular complications resulting from insufficient diabetes control [1]. This disease has also become a challenge for the world of technology. Advances in technology offer the opportunity to improve glycaemic control by reducing the aforementioned complications and burdens while improving quality of life. Closed-loop insulin delivery systems (also known as “artificial pancreas”) take this technology to a larger scale by integrating CGM systems with an insulin pump and an algorithm that automates insulin delivery. These systems are collectively referred to as hybrid closed loop systems (HCL systems). Currently, there are 2 systems: the commercial “auto-mode” and the “Do-it yourself” Artificial Pancreas System (DIYAPS) [2]. The use of technological progress is becoming an important and quite interesting point of care for diabetics, ranging from continuous glucose monitoring systems, through personal insulin pumps, and finally to the use of a combination of both systems to automate the insulin supply [3]. Citing evidence-based medicine, diabetological care in patients with T1D departs from the independent use of the CGM system and the insulin pump, replacing it with the HCL system option [4].
Commercial HCL systems
Closed-loop hybrid systems are characterized by automated algorithm-based insulin delivery and patient-initiated insulin delivery (e.g. post-meal boluses). There are several systems using hybrid closed loop technology currently offered on the global market: Medtronic’s System MiniMed™ 670G, and MiniMed™ 780G (SmartGuard™); Tandem’s T slim x2 Control IQ; Insulet’s Omnipod5Ò-Automated mode (HypoProtect™)[5]; and CamAPS FX DanaRS or Ypso pump [6]. Insulet’s Omnipod5Ò-Automated mode (HypoProtect™) is currently undergoing clinical trials [7]. Technology is moving forward, so advanced systems are being developed that include a developed algorithm with individualization of primary target points, an automated correction bolus function, and improved stability of the automated mode. The automated correction bolus feature is an innovation, which is why we refer to these systems as advanced hybrid closed-loop (AHCL). The AHCL systems include MiniMed™ 780G (SmartGuard™); Tandem’s T slim x2 Control IQ; Insulet’s Omnipod5Ò-Automated mode (HypoProtect™); and CamAPS FX.
The purpose of this paper is to present commercial HCL and AHCL systems devices in 2022 with a scientific perspective.
Discussion
Hybrid closed-loop systems vary; Table 1 [2, 8–39] shows the systems from each manufacturer that are present on the market (2021), allowing us to follow the intentions of the manufacturers, understand their functions, and compare the products. Each system has individual advantages and disadvantages, which we can trace in Table 2 [40, 41]. The imperfections of the systems make the technological progress faster and faster, and this guarantees that newer systems will appear on the market to meet more patients’ expectations. When we look at the advantages of HCL systems we can see many similarities, which are improvements in parameters that reflect diabetes control. The auto-mode algorithms in HCL “learn the patient” and adjust insulin delivery individually for each person. That provides better glycaemic control and safer therapy. However, the existence of different algorithms in systems causes difficulties in interpretation and the need to learn each algorithm. The aforementioned studies indicate that therapy with HCL systems reduces the time spent in hypoglycaemia, while extending the time spent in the target range, aiming to reduce glycaemic fluctuations, and improving the patient’s quality of life in all age groups [42, 43]. Therefore, HCL systems are an essential step forward for patients with diabetes, enabling them to live longer healthier lives and be safer and more comfortable. However, it is important to remember that proper patient education plays a key role in maximizing the benefits of treatment with all HCL systems [22]. For some patients, high-technology systems can add stress and burden, manifesting as switching out of the automated mode when using HCL [2]. Therefore, it is extremely important that technology strives to develop devices that are accessible for use by the common patient, regardless of their socioeconomic status.
Figure 1
Improvement of TIR (%) during therapy using the HCL and AHCL systems in studies [1, 8, 22–32]
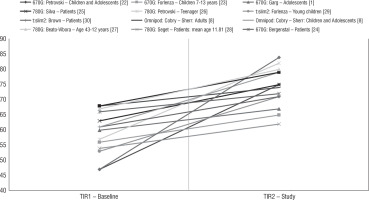
Table I
Characteristics of the commercial HCL and AHCL systems in a table of the research paper by Leelarathna et al. [2], Cobry et al.[8], and Braune et al. [9], with authors’ modification
Table II
Advantages and disadvantages of each system
In addition to focusing on glycaemic control, a broader view is very important in diabetes care, in line with recommendations that include healthy lifestyle medicine, and maintenance of normal blood pressure, body weight, and lipid profile [44, 45, 46, 47]. Interesting issues requiring a separate discussion and allowing for a better therapeutic effect in the use of auto-mode are increasing trust and openness to the new technology of patients and members of the diabetic therapeutic team (percentage of sensor use and change of habits) [48].
Considering the whole, it is an undeniable fact that “auto-mode” systems represent a new stage that can be boldly called a revolution in diabetology.
Conclusions
Technology is moving forward, and with it advances in the treatment of diabetes and the prevention of its complications. The commercial HCL systems presented by the authors give patients with diabetes unique and more satisfactory tools for improving glycaemic control in the real word, but still create a challenge for the patient.

 ENGLISH
ENGLISH






