Introduction
Precocious puberty is a common referral to a paediatric endocrine clinic. The important consequences of precocious puberty include: Compromised final height, early menarche and the psychological impact of maturation on the well-being of the child [1]. Central precocious puberty (CPP) can be idiopathic or neurogenic in nature. Gonadotrophin analogue therapy (GnRHa) has revolutionized the care of girls with precocious puberty [2]. Recent guidelines have dogmatically established the utility of GnRHa therapy in precocious puberty [2, 3]. Review of long term impact on GnRHa therapy on final height of girls with precocious puberty has revealed conflicting results [4]. GnRHa therapy results in suppression of the hypothalamopituitary gonadal (HPG) axis. Recognition and management of children with precocious puberty results in slowing of growth velocity, regression of Tanner’s stage, improvement in uterine size and ovarian volume dimensions and improved final height on a long run [1]. Meticulous assessment of height, body mass index, sexual maturity, growth velocity, bone age, uterine size and ovarian volume dimensions should be done in centres with expertise to optimize long term outcomes [5]. Data on precocious puberty from Indian settings and the impact of therapy on improvement of final height have been published from various Indian centres [6–9]. To the best of our knowledge, there is no data on response of girls with precocious puberty to GnRHa therapy from South India. Hence, we conducted this study to describe the safety and efficacy of GnRHa therapy in girls with central precocious puberty.
Material and methods
We conducted a retrospective review of records of all girls with precocious puberty who were being managed and followed-up in our unit between October 2016 and October 2020. The study was approved by the Institutional review and the scientific committee of the hospital.
Girls with CPP who have received GnRHa therapy for at least one year duration were included. We excluded those with concomitant disorders that can affect growth like central hypothyroidism and growth hormone deficiency (in subjects with tumoral aetiology of central precocious puberty). Usually all girls who presented with onset of thelarche or pubarche before 8 years and menarche before 9 years were referred to the endocrine clinic [10]. Study subjects were evaluated with clinical assessment, bone age assessment, ultrasound to assess uterine size and ovarian volume dimensions and a standard gonadotrophin analogue stimulation test [11]. Sexual maturity of all girls with minimal clothing, in the presence of the mothers were assessed by single clinician and assigned one of the stages described by Tanner [12]. Anthropometric measures (height, weight, body mass index and target height) were measured by standard criteria and converted into Z-scores using appropriate reference standards [13]. Target height was calculated using the formula: Target height = (Father’s height in cm + Mother’s height in cm – 13)/2. The height of both the parents were ascertained in clinic visits.
Bone age was assessed by a singly Pediatric radiologist using the Greulich and Pyle atlas [14]. Predicted final adult height (PAH) was calculated using the Bayley Pinneau (BP) tables [15]. Uterine size, ovarian volume and fundo-cervical ratio was assessed by an ultrasound performed by a single radiologist and converted into Z-scores [16]. Gonadotrophin analogue stimulation test was performed by administration of inj leuprolide in 10 µg/kg sub-cutaneously followed by measurement of LH and FSH at 4 hours, LH, FSH and estradiol at 24 hours. LH, FSH and estradiol were estimated by electro chemiluminescence assay (Roche elecsys cobas e411, USA). The minimum level of detection were 5 pg/ml (18.4 pmol/l) for estradiol and 0.1 mIU/ml for LH and FSH. Girls with either elevated stimulated LH > 5 mIU/l or LH:FSH ratio > 1 are considered as CPP [11]. All girls < 6 years and girls with onset of maturation between 6-8 years who have rapid progression or neurological signs underwent magnetic resonance imaging (MRI) of brain. The cause for central precocious puberty was considered as either idiopathic (normal MRI) or neurogenic (abnormal MRI). Girls who have advanced skeletal maturity (> 2 SD from chronological age) and the risk of early menarche were initiated on GnRHa. Girls were initiated on 12 weekly leuprolide depot injections in doses of: 11.25 mg (in those weighing < 30 kg) and 22.5 mg (in those weighing > 30 kg) [1, 3].
Study subjects were followed up every 3 months in the endocrine clinic. Clinical assessment of Tanner’s stage, presence of any adverse drug reactions like pain, swelling, abscess at the site of injection, headache, nausea, transient vaginal spotting; and anthropometry was assessed in every visit. Clinical and biochemical assessment was done at every 3 monthly visit. LH, FSH and estradiol were measured 3 hours post injection to ascertain adequacy of suppression of hypothalamopituitary Gonadal (HPG) Axis [17, 18]. Ultrasound was done to assess the dimensions of uterus and ovaries 6 monthly, bone age was repeated annually and repeat final height prediction was done based on BP tables.
Statistical analysis
Sample size was estimated using a previous Indian study [6]. The estimate is calculated for comparison of height before and after the administration of intervention. Using mean pre height of 109 cm and post height of 115 cm and a standard deviation of 11.15, for a 5% error (one-sided) and 80% power, the required sample size is 22. The study parameters were tabulated on Microsoft excel. Data is presented as frequencies (percentages) and mean (± standard deviations). Chi-square test, student t-test and paired t-test were used as appropriate. P-value < 0.05 is considered for statistical significance.
Results
During the study period, 39 girls were diagnosed to have CPP. We reviewed the records of 22 girls who satisfied the inclusion criteria and completed gonadotrophin analogue therapy for a mean duration of 2.1 ±0.4 years. The clinical and anthropometric parameters at baseline and end line are described in Table 1. We observed a marginal reduction in height Z-score from 0.4 ±2.2 to 0.1 ±2.3 at the end of the study period (p < 0.05). Parallelly, the BMI Z-score changed from 0.5 ±0.9 to 0.4 ±0.9 to the end of the study period. The biochemical and radiological parameters are described in Table II. Tanner’s staging revealed a regression in the breast growth (40%) and pubic hair (30%). Two children had menarche at the beginning of the treatment which stopped post 3 months of GnRH analogue therapy.
Table I
Comparison of demographic, clinical and anthropometric parameters in study sample at baseline and at the end of the study period a-indicates that p < 0.05
Table II
Comparison of biochemical and radiological parameters in study sample at baseline and at the end of the study period a-indicates p < 0.05
The stimulated LH level reduced from 11.7 ±1.9 to 4.6 ±1.4 mIU/ml (p < 0.05), stimulated FSH level reduced from 16.7 ±2.3 to 6.7 ±3.2 (p < 0.05), stimulated estradiol reduced from 126 ±13.7 to 9.1 ±2.1 pg/ml after initiation of GnRHa therapy. There was a significant reduction in uterine size Z-score, ovarian volume Z-score and fundocervical ratio Z-score at end line versus baseline measurement (Table II).
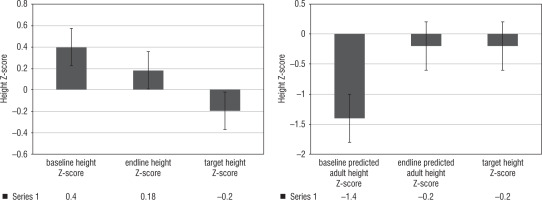
During the study period, the bone age advanced from 7.5 ±2.9 years to 9.5 ± 2.9 years by the end of the study period. The bone age: chronological age (BA/CA) ratio reduced from 1.27 ±0.4 to 1.07 ±0.3 (p < 0.05) (Fig. 1). We observed that there was an increase in predicted adult height from 150.7 ±9.1 cm to 158.6 ±14.1 cm, versus a target height of 157.5 ±6.2 cm (p < 0.05). When it was converted into Z-scores, baseline predicted adult height, end line predicted adult height and target height Z-scores were –1.4 ±0.6, –0.2 ±0.3 and –0.2 ±1.0, p < 0.05, respectively (Fig. 2).
Figure 1
Comparison of Bone age: chronological age ratio in the study sample at baseline and the end of the study period
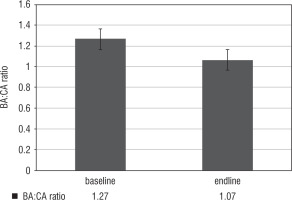
Figure 2
Comparison baseline and endline height Z-scores and predicted adult height Z-scores versus target height Z-scores
Four subjects had neurogenic causes: Pituitary incidentaloma in two cases, low grade glioma in one and hypothalamic hamartoma in one child. All of them were managed conservatively without any surgical intervention. Formal ophthalmological evaluation (including field testing) by a pediatric ophthalmologist were performed in all children, were normal. None of them had any other concomitant pituitary hormone deficiency. Repeat MRI performed in all of them, showed no progress in any of the four subjects. None of the subjects had any significant local or systemic side effects in the period. Two girls experienced transient vaginal spotting that was self-limiting. Increase in dose from 11.25 mg to 22.5 mg was required in one child to improve clinical and biochemical control. Also, two girls who had menarche at onset were initiated on oral medroxyprogesterone acetate 10 mg for a period of 3 months. They did not have any further bleeding episodes. Subjects are being followed up in our endocrine clinic.
Discussion
We have dogmatically demonstrated a good clinical, anthropometric and biochemical and radiological response to GnRHa therapy in our series. In our study we observed a reduction in height Z-scores from 0.4 ±2.2 to 0.1 ±2.3 and a static BMI Z-score from 0.5 ±0.9 to 0.4 ±0.9. This is contrary to a previous study on 32 girls from Turkey where authors observed a statistically significant increase in BMI [19]. We stressed the importance of regular physical activity in every clinic visit and accurately plotted BMI on growth charts and helped the children to maintain their BMI.
Currently, radiologists use three methods to assess bone age and make final height prediction: Tanner white house-3 20 bone method, Tanner white house-3 13 bone method and Greulich and Pyle atlas. Previous reports have indicated that tubular bone maturity is significantly more advanced compared to carpal bone maturity, hence we did not resort to the 20 bone technique [20]. Also, previous reports have indicated that final height prediction by the Bayley Pinneau table is of a good accuracy in majority of girls with central precocious puberty [21]. Also, a South Indian study has established that the Greulich Pyle atlas standards are indeed applicable for south Indian girls [22]. Also, the Greulich Pyle atlas is adopted by our unit for assessment of skeletal maturity. Hence, we used the Greulich Pyle atlas to assess skeletal maturity and Bayley Pinneau tables to make final height predictions in our study. The authors do acknowledge that BP tables are not based on Indian children, but no Indian atlases for bone age estimation and height predictions are currently available. Hence, these girls are being followed up in the endocrine clinic to see whether they attain the final stature as predicted by the BP tables.
The BA/CA ratio reduced from 1.27 ±0.4 to 1.07 ±0.3 (p < 0.05). A similar study from North India showed a retardation in bone age (BA) advancement with a decrease in BA-CA of 1.7 years in girls. The therapy with GnRHa results in control of sex steroid levels which are primarily responsible for the advancement of skeletal maturity in precocious puberty. We have demonstrated an increase in predicted adult height from 150.7 ±9.1 cm (–1.4 ±0.6) to 158.6 ±14.1 cm (–0.2 ±0.3) versus a target height of 157.5 ±6.2 cm (p < 0.05). Three years of GnRHa therapy in subjects with CPP in CAH resulted in an increase in predicted adult height from 142.2 ±6.3 cm vs 150.4 ±4.2 cm, in a study from North west India [7]. Another study on 38 subjects with precocious puberty from North western India resulted in a height SD increment of +0.6 after 3 years of therapy [6].
Our study is not without limitations. The short one year period of follow-up, lack of final height data are important limitations in our study.
To conclude, a good clinical, biochemical and radiological responses was observed to GnRHa therapy in girls with precocious puberty. A significant retardation in progression of bone age and a resultant increase in predicted adult height is demonstrated in our study. Long term follow up is needed to see if these girls attain the final height.

 ENGLISH
ENGLISH






