Introduction
The main cause of acquired hypothyroidism in children is autoimmune thyroiditis (AIT). The incidence of AIT is 2% of the paediatric population, and it is more frequent during adolescence and in females [1]. Biochemical findings usually show high TSH and low T4 levels, and positive titration of thyroperoxidase enzyme (TPOAb) and/or thyroglobulin (TGAb) auto-antibodies. The pathogenetic mechanism is still unknown, even if multifactorial aetiology has been hypothesized including the trigger role of genetic predisposition and environmental factors in susceptible individuals.
Ultrasound imaging is complementary for the diagnosis, and it typically shows a diffuse enlarged thyroid gland, with dishomogeneous echostructure displaying mostly a “pseudonodular” pattern, but sometimes thyroid nodules may be found.
Presenting features of autoimmune hypothyroidism in children may include variable asthenia, gradual scholar performance decrease, slow speech, declining height velocity with short stature, influence in pubertal development, cold intolerance, and constipation.
Typical signs may be observed with weight gain without other major causes, thyroid gland enlargement (goitre), bradycardia, thick dry skin, and sometimes oedematous changes to the soft tissue (myxoedema).
Usually, the disease onset is initially insidious with non-specific features, which may lead to delayed diagnosis if not promptly recognized [2, 3].
Substitutive treatment with levothyroxine (initial dose 1–2 µg/kg/day) is mandatory to solve clinical symptoms and to prevent short- and long-term complications of hypothyroidism.
On 11 March 2020, the World Health Organisation (WHO) declared the infection by the corona (SARS-CoV-2) virus causing COVID-19 disease a global pandemic. Since then, many routine medical care and elective procedures have been postponed due to employment of healthcare practitioners in the emergency setting. Consequently, the SARS-CoV-2 pandemic caused significant changes to healthcare provision throughout the world. Patients faced difficulties accessing facilities for primary care, tertiary referral centre evaluations, as well as diagnostic and therapeutic procedures. Chronic clinical disorders, including hypothyroidism, which are treated in an outpatient setting, have been heavily conditioned by the pandemic scenario [4–9].
There are few data about the potential effects of COVID-19 in thyroid disease presentation in children.
Aim of the study
The aim of this study is to analyse the differences regarding severe autoimmune hypothyroidism onset in children before and during the SARS-CoV-2 pandemic.
Material and methods
The biochemical and clinical data of paediatric patients with autoimmune thyroiditis onset referred to the Department of Paediatric Endocrinology of the Regina Margherita Children Hospital, diagnosed in the period from January 2017 to October 2022, were analysed in a single-centre retrospective observational study. The period from 2017 to 2019 was considered as pre-pandemic, whereas the period from 2020 to 2022 was considered as during the pandemic.
An autoimmune thyroiditis diagnosis was made in the presence of positive titration of TPOAb and/or TGAb. Patients displaying only ultrasound features of thyroiditis were excluded. Severe hypothyroidism was considered when the TSH value was above 100 mUI/l (normal value 0.8–4.8 mUI/l) and fT4 lower than 0.4 ng/l (normal value 0.68–1.3 ng/l). Diagnosis delay was defined as the time elapsed from onset of symptoms to diagnosis of hypothyroidism.
Auxological (weight, height, body mass index, and bone age), clinical (symptoms, growth, and pubertal evolution) and biochemical (TSH, fT4, TPOAb, TGAb) data of patients with severe autoimmune thyroiditis were analysed before treatment start. Levothyroxine requirement was reported for all patients. Nasal-pharyngeal swab for SARS-CoV-2 detection was performed in all patients at hypothyroidism diagnosis.
Thyroid ultrasound evaluation was performed in all patients at disease onset. The volume of the thyroid gland was calculated using the formula: volume = 0.5 × (length × anterior-posterior depth × transverse width), and the results were expressed in SDS (individual thyroid volume × mean thyroid volume for age/ SD) according to the reference values provided by Vitti et al. [10].
Pituitary MRI was performed in all patients with severe hypothyroidism, to rule out pituitary hyperplasia or autonomous TSH-secreting adenoma. In patients with pituitary hyperplasia, ACTH, cortisol, prolactin, and somatomedin-C were measured. In patients presenting short stature, a dynamic test for GH secretion was also performed, once the thyroid biochemical profile result was normal.
Statistical analysis was performed using GraphPad 7 Software (GraphPad Software, La Jolla, CA, USA) by the Mann-Whitney U test to compare the means between groups.
Results
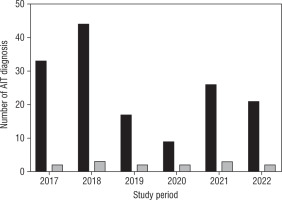
In the study period a total of 150 subjects with autoimmune thyroiditis onset were enrolled. Of these, 94 were diagnosed in the pre-pandemic period, and 56 during the pandemic. Severe hypothyroidism was detected in 14 patients (7/94 in the pre-pandemic period [7.5%] and 7/56 during the pandemic period, [12.5%]) (Figure 1). As shown in Figure 1, during the pre-pandemic period, 2/33 (6.1%) subjects were diagnosed as severe hypothyroidism in 2017, 3/44 (6.8%) in 2018, and 2/17 (11.8%) in 2019. During the SARS-CoV-2 global emergency, 2/9 (22.2%) subjects with severe hypothyroidism were diagnosed in 2020, 3/26 (11.5%) in 2021, and 2/21 in 2022 (9.5%). All patients diagnosed during the pandemic period were SARS-CoV-2 negative.
Figure 1
Epidemiological distribution of severe AIT compared to total AIT onset before (2017–2019) and during the pandemic years (2020–2022)
Auxological and biochemical data at the onset of severe autoimmune hypothyroidism are reported in Table I.
Table I
Auxological and biochemical data at severe hypothyroidism onset
Mean age at the onset in the pre-pandemic period was lower with respect to subjects with severe AIT onset during the pandemic period (p = 0.04).
Diagnosis delay was significantly different between the before and during the pandemic groups (p = 0.02).
In the pre-pandemic period the mean TSH value at onset was 447.7 ±59.1 mUI/l, and it was 713.7 ±104.4 mUI/l during the SARS-CoV-2 pandemic (p = 0.04).
At the onset the mean fT4 value was 2.66 ±0.34 ng/l in the pre-pandemic period, whereas it was 0.58 ±0.08 ng/l during the pandemic (p = 0.0002).
Significantly greater thyroid volume and bone age delay SDS were observed during the pandemic period (p = 0.04).
At MRI evaluation pituitary hyperplasia was detected in 1/7 and 5/7 subjects before and during the pandemic period, respectively (p = 0.03). Among all subjects with pituitary hyperplasia, only one subject, during the pandemic period, was diagnosed with growth hormone deficiency.
No difference was observed in gender, levothyroxine requirement, growth, and pubertal retardation among the before and during the pandemic period groups. No other autoimmune disorders were observed in the groups.
Symptoms at the onset of severe AIT are represented in Table II. Neurological symptoms were mostly observed during the pandemic period, especially slow speech and impaired scholar performance.
Table II
General and neurological symptoms at severe AIT onset in before and during the pandemic period
Discussion
Autoimmune thyroiditis in children may present with insidious onset, and usually with non-specific clinical features. This may lead to delayed diagnosis if not promptly recognized, even in cases of severe hypothyroidism.
The SARS-CoV-2 global emergency has radically changed access in primary health care services, especially if delivered in an outpatient setting.
Several studies have evaluated the effects of COVID-19 disease in thyroid disorders. The British Thyroid Association and the American Thyroid Association state that patients with hypothyroidism do not present greater risk of COVID-19 infection nor a more severe disease course [11, 12]. Also, currently there is no evidence that the SARS-CoV-2 virus may affect the function of the thyroid gland [13, 14]. Debatable data have been reported in adults about the correlation between viral infection and the risk of developing thyroid disease [15]. Also, thyroid function monitoring is recommended [16, 17]. Few data are available about the effect of the SARS-CoV-2 virus on thyroid disorders in childhood. McCowan et al. did not observe a significant difference in the presentation of thyroid dysfunction in children before and during the pandemic period [5]. Shidid et al. did not report any difference in the percentage of abnormal TSH tests in childhood in the 2 periods [6]. This can be partly explained by the lower spread rate of SARS-CoV-2 infection among the paediatric population [8].
Contrary to adults, in children there was not an increase in onset of autoimmune disorders in the post-pandemic years [8].
In our study we analysed the effect of the pandemic emergency on severe autoimmune thyroiditis (AIT) onset. We observed a higher rate of severe AIT onset during the pandemic period (12.5% vs. 7.5%), even if overall autoimmune thyroiditis diagnoses were lower (56 vs. 94). This latter may be explained by the missed diagnosis of mild autoimmune thyroiditis, related to the lower rate of thyroid function testing performed due to the pandemic scenario.
We found higher time lapse from onset of symptoms to diagnosis of severe hypothyroidism in the subjects during the pandemic period. This may explain the significant difference in age of presentation between the 2 groups. Higher TSH value, lower fT4 level, and greater thyroid volume were observed in the subjects diagnosed during the post-pandemic years, even if levothyroxine daily requirement was not significantly different. Bone age delay and pituitary hyperplasia were also detected more frequently during the post-pandemic period. At disease onset all subjects presented with asthenia. Cold intolerance was found to be more frequent in the pre-pandemic period, whereas weight gain was mostly observed in the subjects receiving diagnosis during the SARS-CoV-2 emergency. At presentation, neurological symptoms were mostly observed during the pandemic period, especially slow speech and impaired scholar performance, probably related to the diagnosis delay.
The limitations of the study are the retrospective data analysis, the small cohort of children affected by severe hypothyroidism, and the lack of longitudinal data about the growth and puberty outcomes of patients with AIT onset before and during the pandemic emergency. Diagnosis delay evaluation could be partly biased by the non-specific symptoms of hypothyroidism onset. The lack of standardized tests regarding neurological assessment may have influenced the results, even if in all cases the onset of reported neurological symptoms was prior to the pandemic period.
Conclusion
This single-centre retrospective study in a tertiary centre of Paediatric Endocrinology revealed a higher rate of severe autoimmune hypothyroidism during the pandemic period, mostly related to the radical change in the daily behaviour of the population and the government restrictions, which led to difficulties in access to healthcare services. The diagnosis delay was related to the severity of biochemical thyroid hormone profile at the onset, as well as with goitre volume, bone age delay, and more frequent neurological symptoms. These findings confirm again the fundamental importance of recognizing hypothyroidism symptoms in childhood, even if often non-specific, and the necessity to perform thyroid hormone profile evaluation in cases of symptoms that may recall hypothyroidism.

 ENGLISH
ENGLISH






