Introduction
Diabetes mellitus (DM) is a chronic, common, and multifactorial disease that presents with hyperglycaemia as the primary manifestation, as well as polyuria, polydipsia, starvation, weight loss, fatigue, and weakness [1]. Diabetes mellitus is divided into 4 main categories: type 1 diabetes mellitus, type 2 diabetes mellitus, gestational diabetes, and other specific types [1]. Type 1 diabetes mellitus (T1DM), or insulin-dependent diabetes mellitus (IDDM), accounts for 5–10% of cases and is usually diagnosed in childhood or adolescence [2]. It is the second most common autoimmune disease in children and is associated with high mortality and morbidity throughout life [3]. Annually, about 86,000 new cases of the disease are identified, and the incidence of T1DM increases by about 3% per year [2]. Genetic and environmental factors play an important role in the development of T1DM [4]. The environmental factors include lifestyle changes, diet, health, consumption of antibiotics, viral infections, type of infant feeding (lack of breastfeeding and early feeding of cow’s milk and gluten), and lack of intake or production of vitamin D [4,5]. Gastrointestinal bacteria are an important factor in the pathogenesis of T1DM [4]. These bacteria and their metabolites affect the function of the intestinal mucosa and autoimmunity against β cells of the pancreas [4]. Disruption of the intestinal bacterial flora affects the metabolism of glucose, lipids, and insulin activity, and leads to metabolic syndrome, insulin resistance, and diabetes [6]. Animal studies show that an imbalance in intestinal microflora leads to increased production of lipopolysaccharides and inflammation of the intestinal mucosa, resulting in decreased connectivity between intestinal epithelial cells and increased intestinal permeability. The infiltration of lipopolysaccharides from the intestinal epithelium into the bloodstream causes systemic inflammation, insulin resistance, and poor blood glucose control [7]. Studies have shown that the intestinal bacteria differ between diabetic and non-diabetic individuals, with a decrease in the strains of phylum Firmicutes and Clostridia, and an increase in the strains of Bacteroidetes and Proteobacteria [8]. Probiotics are living microorganisms that can have beneficial effects on the balance of intestinal microflora and the health of the host. Common strains of the probiotics are Lactobacillus, Bifidobacterium, and Saccharomyces bulardii, which have different effects depending on the amount, duration, and method of use [9]. Recent studies have shown that probiotics can promote the growth of beneficial microorganisms in the gut and improve the course of diabetes [6, 10, 11]. The effectiveness of probiotics in the treatment of some diseases such as obesity, insulin resistance, type 1 and 2 diabetes, and non-alcoholic fatty liver has been reported [12]. Several mechanisms have been proposed regarding the positive effects of probiotics on glycaemic status. Probiotics may affect the intestinal microbiota to enhance the production of glucagon-like peptides and insulinotropic peptides, which cause more glucose uptake by tissues such as muscles and liver [11, 13]. On the other hand, probiotics lead to the improvement of intestinal defence barrier function and intestinal integrity by increasing the number of Gram-positive bacteria in the intestine and has immunomodulatory effects [14–16]. Some strains of probiotics are involved in acetate production. Acetate regulates the function of the immune system; therefore, it has beneficial effects on T1DM [17].
Purpose
The aim of this study was to evaluate the effectiveness of oral probiotics on glycosylated haemoglobin (HbA1c) levels in children with T1DM and the process of glycaemic control.
Material and methods
Study design: This study was designed as a single-blind randomized clinical trial. Children aged 2–16 years with T1DM referred to the endocrine clinic of Bahrami Children’s Hospital during one year (2018–2019) were enrolled.
Inclusion criteria including:
Proof of T1DM by history, interview, and information of his/her medical record.
Onset of the disease within the last 3 years.
No history of proven congenital or acquired immunodeficiency.
Exclusion criteria including:
Patients consuming probiotics in the last 4 weeks.
Patients consuming antibiotics in the last 2 weeks.
Gastrointestinal infections in the last 2 weeks.
Presence of chronic underlying intestinal diseases.
Presence of celiac disease.
Sample size and selection of the groups: The total sample size of 52 was calculated (including 26 patients in each group) based on the following formula and with an α default of 0.05 (95% confidence level), a β default of 20% (80% power), and an acceptable error rate of 0.2.
Randomization and blinding: To randomize the sampling, a computer number determinant was used. It performed in a ratio of 1:1 for the probiotic or control group. For single blinding of the study, participants in both groups (probiotic and control) were kept blind. The probiotic group, in addition to the routine treatment of diabetes (insulin therapy), also received probiotic capsules. This group did not know the type of drug (blinding in the probiotic group). The control group received only routine treatment (insulin therapy) and remained unaware of the intervention in the other group (blinding in the control group without placebo).
Intervention: Eligible children entered the study after written consent from themselves and their parents. Patients were classified into 2 groups. The first group was the intervention group, which, in addition to the appropriate treatment for diabetes (insulin), received one probiotic capsule daily for 3 months (named Prokid, GostareshMilad Pharmed company, made in Iran), containing various strains of non-pathogenic microflora including Bifidobacteriumlactis over 5.3 billion, Bifidobacteriumbifidum over 1 billion, Lactobacillus acidophilus over 1 billion, and Lactobacillus ramenus over 5 billion. This group was named the probiotic group. By direct communication with the parents of diabetic children in the probiotic group, the assurance of probiotic consumption was monitored. The second group was the control group, who did not receive any additional treatment other than insulin.
Diabetes duration in both groups was between 6 months and 3 years, and there was no sharp difference between the 2 groups. The regimen of insulin consumption used in the 2 groups was the same: total daily insulin dose for patients under 7 years old was 0.3–0.6 units per kg of body mass, and total daily insulin dose for patients above 7 years old was 0.7–1 units per kg of body mass. The portion of basal insulin was 40–60% of the total daily insulin. The kind of insulin administration was multiple daily injections (MDI) in all patients, and the type of insulin used was analogue insulin. In both groups, insulin therapy was delivered in the usual way, and no patients receives additional HCP support. Also, insulin therapy was performed in both groups based on reaching the target blood glucose range.
The diet of children in both groups was determined to be diabetic, which is appropriate for type 1 diabetes. The fasting venous blood samples (10 hours fasting) were taken at the beginning and end of the study to measure fasting plasma glucose (FPG), glycated haemoglobin (HbA1c), cholesterol (Chol), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG). Finally, the results were compared.
End points
The primary end point was glycated haemoglobin (HbA1c) response to probiotics.
The secondary end points included fasting plasma glucose (FPG), cholesterol (Chol), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG).
Ethical considerations
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (ethics committee code: IR.TUMS.Medicine.rec.1396.4356) and was registered in the Clinical Trial Registration Centre of Iran with the code IRCT20180128038534N1 on 2018-06-13.
Statistical analysis methods
Data were analysed using SPSS software (version 22). The mean was used to express quantitative variables, and the frequency was used to express qualitative variables. A paired t-test was used to compare the mean of the biochemical variables before and after the intervention.
Results
A total of 52 patients were included. They were classified in 2 groups of 26 individuals each. Ultimately, all of the patients completed the study. These are demonstrated in Figure 1. The mean age of children was 9.3 ±2.9 (4 to 14) years. The mean age in the probiotic and control groups was 9.6 ±3.5 and 9.4 ±3.0, respectively. There was no significant difference between the 2 groups in terms of age (p-value > 0.05). Twenty-seven patients (52%) were male, of whom 14 (54%) and 13 (50%) were in the probiotic and control groups, respectively. The results of lab tests before and after the intervention are summarized in Tables I and II.
Table I
Lab tests before and after the intervention
Table II
Comparison of lab tests before and after the intervention in the probiotic and control groups
Before the intervention, there were no statistically significant differences between the probiotic and control groups in terms of FPG, HbA1c, TG, cholesterol, LDL, HDL, and BMI. At the end of the study, the mean percentage of HbA1c was higher in both groups (Figure 2); however, the increase in HbA1c in the probiotic group (increase of 0.1%) was clearly lower than in the control group (increase of more than 0.4%). This decrease in HbA1c rising in the probiotic group was not statistically significant (p = 0.692). Therefore, it cannot be concluded that the effect of probiotic consumption on reducing HbA1c was significant.
Figure 2
Comparison levels of HbA1c in the probiotic and control groups before and after the intervention
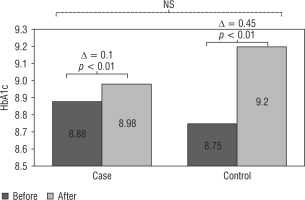
Figure 3
Comparison of FPG levels in the probiotic and control groups before and after the intervention
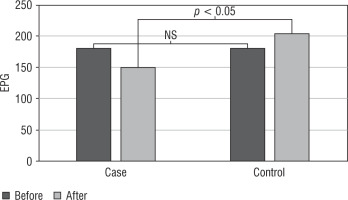
As seen in Table II, the mean level of FPG was significantly lower in the probiotic group than in the control group (p = 0.016). Comparing the groups in terms of lipid parameters, there was a decrease in total cholesterol and LDL levels in the probiotic group; however, this decrease was not significant (respectively, p = 0.920 and p = 0.597). Also, there was an increase in the HDL and triglyceride levels in the probiotic group, but this increase was also not significant (respectively, p = 0.684 and p = 0.500).
Discussion
In this study, we investigated the effect of probiotics on HbA1c in children with T1DM. It was observed that the use of probiotics can have beneficial effects on biochemical markers. During the study, acute complications of diabetes, like ketoacidosis or considerable hypoglycaemia, did not occur in the studied patients. The mean percentage of HbA1c was high in both groups after intervention, but the increase in HbA1c in the probiotic group (increase of 0.1%) was less than in the control group (increase of more than 0.4%). This decrease in HbA1c rising in the probiotic group was not statistically significant (p = 0.692). Compared with the control group, the mean level of FPG was significantly lower in the probiotic group. Although in the results we obtained in this study probiotic consumption did not cause a significant reduction in HbA1c in the probiotic group, it did cause a significant decrease in FPG in the probiotic group. All in all, these findings suggest the possibility of beneficial effects of probiotics on the glycaemic status of patients with T1DM, but there is a need for more research in this regard. The insulin dose in both groups before the intervention was as follows: the total daily insulin dose for patients under 7 years old was 0.3–0.6 units per kg of body mass, and the total daily insulin dose for patients above 7 years old was 0.7–1 units per kg of body mass. According to the results of our study, probiotics did not have a clear effect on the HbA1c of the studied cases. So, after the intervention, the total daily dose of insulin in both groups continued at the same level as before.
In several studies, the beneficial effects of probiotics on autoimmune diseases have been investigated. This issue is important in type 1 diabetes patients because the aetiology of this disease is the dysfunction of the immune system, and in the process of this disease there is a possibility of involvement of other internal organs by the immune system [18, 19].
The effectiveness of probiotics in diabetic patients has been observed in some animal and human studies, most of which have been in T2DM. There are few research papers on the effect of probiotics on T1DM, especially in children. An animal study depicted that administration of oral Lactobacillus GG in rats with diabetes (secondary to streptozotocin) can lower HbA1c, and improve glucose tolerance and insulin resistance [20]. Ahmadian et al. showed that probiotic use in adults with type 2 diabetes reduced the levels of blood glucose and insulin resistance. They used a daily dose of 109 CFU probiotics including several strains of Lactobacillus (acidophilus, casai, bulgarigus, rhamnosus), several strains of Bifidobacterium (Logum, Brev), and Streptococcus thermophilus [21].
We also investigated the effect of probiotics on the blood lipid profile. In our study, there was a decrease in total cholesterol and LDL levels in the probiotic group; however, this decrease was not significant (respectively, p = 0.920 and p = 0.597). Also, there was an increase in the HDL and triglyceride levels in the probiotic group, but this increase was also not significant (respectively, p = 0.684 and p = 0.500).
Comparison with previous studies
Based on our research in the literature, there are very few studies evaluating the effect of probiotics on children with T1DM, and most of these studies are on adults with type 2 diabetes. The only study of our interest was that of Groele et al., which evaluated the effect of probiotics on β-cell function in children with type 1 diabetes. Their results were similar to our study regarding the effect on HbA1c [5].
One of the strengths of our study was the careful and regular use of probiotics in the probiotic group and stringent monitoring of patients in both groups. The limitation was the limited number of participants. Therefore, future studies with more patients and longer duration are needed.
Conclusion
According to the results of our study, consumption of oral probiotics has no significant effect on HbA1c levels in children with type 1 diabetes mellitus. Another result of this study was a significant decrease in the mean level of FPG in the probiotic group after the intervention, so this issue may indicate the potential of future studies on the beneficial effect of probiotics in relation to glycaemic status in diabetic children with T1DM.

 ENGLISH
ENGLISH