Introduction
Congenital hypothyroidism (CH) is estimated to affect about one in 1500 to 4000 children (in the Polish population one in 3500) [1–4]. That makes it one of the most common congenital endocrine disorders in childhood. In the majority of cases (65–85%) it is caused by alterations during the gland organogenesis, resulting in thyroid agenesis (athyreosis), thyroid hypoplasia, or thyroid ectopy [6, 5]. In the remaining cases, CH is caused by a congenital defect of thyroid hormone biosynthesis, secretion, or recycling. The dyshormonogenesis is inherited in an autosomal recessive pattern, while most cases of thyroid dysgenesis are sporadic. Nevertheless, mutations in several genes that regulate thyroid gland development have been reported as rare causes of thyroid dysgenesis (PAX8, TTF2, NKX2-1, NKX2-5, GLIS3, JAG1, CDCA8, TPO), and only 2% of cases with thyroid dysgenesis are found to have such genetic mutations [8, 9]. Recently, BOREALIN and DUOX2 gene mutations have also been reported in patients with thyroid ectopy [3, 6]. This genetic heterogeneity is probably responsible for the variable clinical course and differences in the function and size of the thyroid gland [3]. The ectopic thyroid was first described by Hickman in 1869 in a newborn who was suffocated 16 hours after birth because of a lingual thyroid causing upper airway obstruction [10]. In the neonatal period, thyroid ectopy may be accompanied by symptoms such as acute respiratory distress, stridor, and poor feeding [11], leading to further sleep apnoea and growth restrictions [12, 13]. The thyroid gland begins to form approximately 24 days after fertilization from an outgrowth of the pharyngeal endoderm. As the embryo grows, the thyroid gland descends into the neck to its final position by the seventh week of gestation. This migration of thyroid cells can give rise to ectopic thyroid tissue. The most common sites of ectopic thyroid tissue occur along this migration path and include the tongue, larynx, trachea, oesophagus, mediastinum, heart, and abdomen [7]. Thyroid dysgenesis is not always associated with hypothyroidism; however, in approximately 70% of children with ectopic thyroid, subclinical or overt primary hypothyroidism may develop [14].
Case presentation
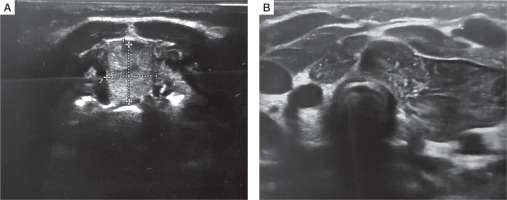
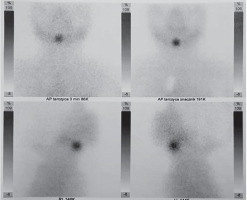
A female infant aged 3 months was admitted to the outpatient endocrine clinic due to suspicion of primary hypothyroidism. The TSH level in a random venous blood sample was 28 µIU/ml. The child was born at 38 weeks of gestational age by caesarean section, from the second pregnancy of nonconsanguineous parents. During pregnancy the mother was receiving levothyroxine in a dose of 50 µg/day due to elevated TSH level. Birth weight was 3700 g (0.97 SD), length was 57 cm (4.22 SD), and head circumference was 33 cm (–0.3SD). Apgar score was 10 in the first minute. The first result of a screening test for TSH was slightly above the upper limit of the normal range (TSH 19.94 µIU/ml; N < 18 µIU/ml), but when repeated on 6th day it was normal (9.96 µIU/ml; N: < 12 µIU/ml). Postpartum the mother started to breast feed. There was no need to continue the levothyroxine supplementation. An additional TSH test was performed because of the mother’s concerns about the previous positive result. At admission the body weight 7.1 kg, which was proper for body length (67 cm), both above the 97th percentile. The anterior fontanelle’s size was 1 × 1 cm. The heart rate was 110 bpm. The child did not present any signs of abnormal thyroid function. The neurological development was appropriate for the child’s age. No infection occurred during the first 3 months of life. The diagnosis of primary subclinical hypothyroidism was established on the basis of a blood test performed in the endocrine clinic: TSH 26.3 µIU/ml (N: < 10 µIU/ml), with FT4 14.7 pmol/l (N: < 10–25 pmol/l) and fT3 6.9 pmol/l (N: 3–8 pmol/l), and Tg (thyroglobulin) 455 ng/ml (N: 3.5–77 ng/ml). Neck ultrasonography (US) revealed ectopically located thyroid tissue in the submandibular area (Fig. 1). Scintigraphy with technetium-pertechnetate (99mTc) confirmed submandibular thyroid gland with empty fossa (Fig. 2). The treatment with liquid levothyroxine in the daily dose of 2 µg per kg of body weight was introduced. The control examination after 2 weeks revealed the normalization of TSH level (7.56 µIU/ml) with fT4 (16.7 pmol/l), and fT3 (6.9 pmol/l) within normal range. In the US control after one year, the dimensions of the ectopic thyroid gland decreased (11 × 12mm), and morphology and biochemical tests were within normal range. At 12 months of age the child showed normal psychomotor development. The dose of the levothyroxine was 3 µg/kg/day.
Discussion
Newborn screening for congenital hypothyroidism (CH) has been highly effective in preventing serious neurodevelopmental and physical consequences in affected children. In Poland the screening procedure is based on TSH level measurement in a dried blood spot taken from a newborn in the first days of life [4]. The normal value for full-term newborns is defined as TSH < 12 mIU/l. In the case of TSH level ≥ 12 mIU/l and < 28 mIU/l the test has to be repeated according the Newborn Screening Programme for Poland 2019–2022 [4]. In the case of TSH level ≥ 28 mIU/l the neonate has to be admitted to a reference centre to confirm the diagnosis by measuring the levels of TSH and fT4 in the venous blood [4]. A similar screening model is used in many countries, but cut-off values vary with the population [3]. However, screening has some limitations. It does not allow for the differentiation of the cause of hypothyroidism. Also, some cases of ectopic thyroid can be missed in neonatal screening, giving a false negative result because it initially produces sufficient hormones, which decrease during early infancy and childhood [15]. That may lead to irreversible disorders in the development of the nervous system and to developmental delay. That is why some researchers suggest supplementary diagnostic tests, especially in doubtful cases [18, 19]. Some studies also indicate the usefulness of the assessment of the thyroglobulin level in the blood as useful information about the presence or absence of functional thyroid tissue [16]. This examination, however, is of little value in the case of ectopy of the thyroid gland with partially preserved function. Between 2002 and 2005 Iranpour et al. used scintigraphy with Tc-99m as an additional screening test for second-recalled newborns before thyroxin replacement therapy. They revealed with that additional thyroid scan that 28.4% of infants were athyreotic, 6% had ectopic thyroid, and 65.6% had a normal thyroid [17]. The study confirmed that Tc-99m TS is a useful diagnostic tool in thyroid dysgenesis. However, because it is not always possible to perform this examination, it seems justified to perform at least the US examination in doubtful cases because it is accessible and not burdensome. In congenital hypothyroidism, US examination is the primary method for distinguishing orthotopic from ectopic thyroid [18]. In approximately 20% of infants with a re-evaluated screening test, thyroid scintigraphy and/or US revealed a structural defect [19]. In any doubtful results of the neonatal screening test, or in any case of suspected congenital hypothyroidism, the diagnosis should be supplemented with non-invasive ultrasound examination of the neonate’s neck, and followed by scintigraphy if necessary.

 ENGLISH
ENGLISH